Equillium Announces Positive Data from Phase 2 Study Evaluating Itolizumab in Patients with Moderate to Severe Ulcerative Colitis
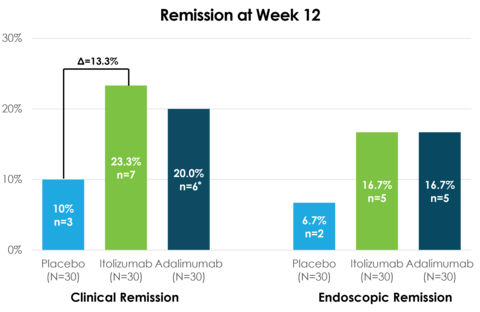
Itolizumab demonstrated clinical efficacy after 12 weeks of treatment, achieving a clinical remission rate of 23.3% compared to 20.0% for adalimumab and 10.0% for placebo
Itolizumab achieved key secondary endpoint of endoscopic remission of 16.7% compared to 16.7% for adalimumab and 6.7% for placebo
Itolizumab was generally well tolerated consistent with prior clinical experience
LA JOLLA, Calif.–(BUSINESS WIRE)–Equillium, Inc. (Nasdaq: EQ), a clinical-stage biotechnology company leveraging a deep understanding of immunobiology to develop novel therapeutics to treat severe autoimmune and inflammatory disorders, and Biocon Limited (BSE code: 532523, NSE: BIOCON), an innovation-led global biopharmaceutical company, today announced positive topline results from the Phase 2 study evaluating itolizumab in the treatment of moderate to severe ulcerative colitis (UC).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250206241338/en/
(Graphic: Business Wire)
The double-blinded, placebo- and active-controlled Phase 2 clinical study evaluated the safety and efficacy of itolizumab in biologic-naïve patients with moderate to severe active UC. A total of 90 patients were randomized 1:1:1 to receive itolizumab (fixed dose of 140 mg), placebo, or adalimumab (a global standard of care biologic treatment used as an active control) every two weeks for an initial 12-week treatment period. The primary endpoint of the study was clinical remission as defined by Total Mayo Score, and secondary endpoints included the proportion of participants who achieved clinical response and endoscopic remission (evaluated by central endoscopy). The study was co-sponsored by Equillium and Biocon Limited and conducted at multiple clinical trial sites in India. The design and conduct of the trial were a collaborative effort, with input from the gastroenterology community and leading global clinical and scientific experts in the field of inflammatory bowel disease (IBD).
“The CD6-ALCAM pathway is elevated in gastrointestinal inflammation and is associated with severity of disease in both ulcerative colitis and Crohn’s patients. As such, we are delighted with the strength of data across the primary and secondary endpoints of this Phase 2 study in moderate to severe ulcerative colitis patients,” said Dr. Stephen Connelly, chief scientific officer at Equillium. “Itolizumab was well tolerated and achieved a clinical remission rate of 23 percent despite an imbalance of more severe patients in the itolizumab arm compared to the other arms of the study. While these positive results add to itolizumab’s critical mass of safety and efficacy data across different patient populations, we are particularly encouraged by this data in the context of our Phase 3 EQUATOR study in acute graft-versus-host disease, where lower gastrointestinal pathogenesis is a key driver of mortality, with topline data expected this quarter.”
“Itolizumab demonstrated proof of concept with a meaningful effect size – comparable to biologic standard of care adalimumab – in this Phase 2 study in subjects with moderate to severe ulcerative colitis,” said Dr. Brian Feagan, Professor of Medicine at the Schulich School of Medicine & Dentistry at the University of Western Ontario. “Itolizumab represents a novel selective immune modifying mechanism of action with great potential in a treatment paradigm needing differentiation and improved outcomes for patients.”
Summary of Key Study Results
Baseline demographics of the study included a median age of 39 years, relatively equal proportions of male and female subjects evenly distributed among study arms, and a mean weight of 58 kilograms.
Baseline disease severity of the study was greater in the itolizumab arm, where 23% of patients were classified as severe (Total Mayo Score of 11) versus 0% in the placebo and adalimumab arms, and 66% had left-sided colitis versus 30% and 43% in the placebo and adalimumab arms, respectively.
The primary endpoint of the study was clinical remission, defined as Total Mayo Score of ≤ 2 with no individual sub-score greater than 1 at Week 12. Secondary endpoints included the proportion of participants who achieved clinical response (per Total Mayo Score) and endoscopic remission (evaluated by central endoscopy) at Week 12 and Week 24. Additional data is expected to be presented at a future scientific conference during 2025.
- 23.3% clinical remission in the itolizumab arm at 12 weeks (primary endpoint) vs. 20.0% in adalimumab* vs. 10.0% in placebo
- 63.3% clinical response in the itolizumab arm at 12 weeks vs. 60.0% in adalimumab vs. 46.7% in placebo
- 16.7% endoscopic remission in the itolizumab arm at 12 weeks vs. 16.7% in adalimumab vs. 6.7% in placebo
- Itolizumab was generally well tolerated, and no safety signal was observed
* One patient from the adalimumab arm had ‘endoscopic remission,’ but was recorded as ‘not in clinical remission’ at week 12 due to missing sub-score data
About Itolizumab
Itolizumab is a clinical-stage, first-in-class immune-modifying monoclonal antibody that selectively targets the CD6-ALCAM signaling pathway to downregulate pathogenic T effector cells while preserving T regulatory cells critical for maintaining a balanced immune response. This pathway plays a central role in modulating the activity and trafficking of T cells that drive a number of immuno-inflammatory diseases.
The blockade of CD6 with itolizumab has demonstrated a reduction in T effector cell proliferation and downregulation of several important pathways that contribute to T effector cell development. The downregulation of these pathways is accompanied by decreased secretion of the pro-inflammatory T effector cytokines IFN-γ, TNF-α, IL-6 and IL-17. Additionally, inhibiting the binding of ALCAM to CD6 modulates lymphocyte trafficking and results in reduced T effector cell infiltration into inflamed tissues.
About Biocon Limited
Biocon Limited, publicly listed in 2004, (BSE code: 532523, NSE Id: BIOCON, ISIN Id: INE376G01013) is an innovation-led global biopharmaceuticals company committed to enhance affordable access to complex therapies for chronic conditions like diabetes, cancer and autoimmune. It has developed and commercialized novel biologics, biosimilars, and complex small molecule APIs in India and several key global markets as well as Generic Formulations in the US, Europe & key emerging markets. It also has a pipeline of promising novel assets in immunotherapy under development. Website: www.biocon.com; Follow-us on Twitter: @bioconlimited for company updates.
About Equillium
Equillium is a clinical-stage biotechnology company leveraging a deep understanding of immunobiology to develop novel therapeutics to treat severe autoimmune and inflammatory disorders with high unmet medical need. The company’s pipeline consists of the following novel first-in-class immunomodulatory assets and product platform targeting immuno-inflammatory pathways. Itolizumab: a monoclonal antibody that targets the CD6-ALCAM signaling pathway which plays a central role in the modulation of effector T cells that drive a number of immuno-inflammatory diseases. It is currently under evaluation in a Phase 3 clinical study of patients with acute graft-versus-host disease (aGVHD) and has exhibited positive data from both a Phase 2 clinical study of patients with moderate to severe ulcerative colitis and a Phase 1b clinical study of patients with lupus/lupus nephritis. Equillium acquired rights to itolizumab through an exclusive partnership with Biocon Limited, who also provides commercial manufacturing for the product. EQ101: a selective tri-specific cytokine inhibitor targeting IL-2, IL-9, and IL-15, has exhibited positive results in both a Phase 2 proof-of-concept clinical study of patients with moderate to severe alopecia areata and a Phase 1/2 proof-of-concept clinical study of patients with cutaneous T cell lymphoma (CTCL). EQ302: an orally delivered, selective bi-specific cytokine inhibitor targeting IL-15 and IL-21 at pre-clinical stage.
For more information, visit www.equilliumbio.com.
Forward-Looking Statements
Equillium
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words such as “anticipate”, “believe”, “could”, “continue”, “expect”, “estimate”, “may”, “plan”, “outlook”, “future”, “potential” and “project” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These statements include, but are not limited to, statements regarding Equillium’s plans and strategies with respect to developing itolizumab, the encouraging impact of the positive data in the context of Equillium’s Phase 3 EQUATOR study in aGVHD, the expected timeline for the presentation of additional data from the Phase 2 study of itolizumab in UC, and the potential benefits of Equillium’s product candidates. Because such statements are subject to risks and uncertainties, many of which are outside of Equillium’s control, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: Equillium’s ability to execute its plans and strategies; risks related to performing clinical and pre-clinical studies; whether the results from clinical and pre-clinical studies will validate and support the safety and efficacy of Equillium’s product candidates; changes in the competitive landscape; and changes in Equillium’s strategic plans. These and other risks and uncertainties are described more fully under the caption “Risk Factors” and elsewhere in Equillium’s filings and reports, which may be accessed for free by visiting the Securities and Exchange Commission’s website and on Equillium’s website under the heading “Investors.” Investors should take such risks into account and should not rely on forward-looking statements when making investment decisions. All forward-looking statements contained in this press release speak only as of the date on which they were made. Equillium undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Biocon
This press release may include statements of future expectations and other forward-looking statements based on management’s current expectations and beliefs concerning future developments and their potential effects upon Biocon and its subsidiaries/ associates. These forward-looking statements involve known or unknown risks and uncertainties that could cause actual results, performance or events to differ materially from those expressed or implied in such statements. Important factors that could cause actual results to differ materially from our expectations include, amongst other: general economic and business conditions in India and overseas, our ability to successfully implement our strategy, our research and development efforts, our growth and expansion plans and technological changes, changes in the value of the Rupee and other currency changes, changes in the Indian and international interest rates, change in laws and regulations that apply to the Indian and global biotechnology and pharmaceuticals industries, increasing competition in and the conditions of the Indian and global biotechnology and pharmaceuticals industries, changes in political conditions in India and changes in the foreign exchange control regulations in India. Neither Biocon, nor our Directors, nor any of our subsidiaries/associates assume any obligation to update any particular forward-looking statement contained in this release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250206241338/en/
Corporate Contact
Michael Moore
Vice President, Investor Relations & Corporate Communications
619-302-4431
[email protected]
Biocon Limited
Saurabh Paliwal
Head – Investor Relations
+91 9538380801
[email protected]
Biocon Limited
Calvin Printer
Head – Corporate Communications
+91 7032969537
[email protected]
KEYWORDS: California United States India North America Asia Pacific
INDUSTRY KEYWORDS: Health Health Technology Clinical Trials General Health Pharmaceutical Biotechnology
MEDIA:
| Photo |
 |
| (Graphic: Business Wire) |
| Logo |
 |
