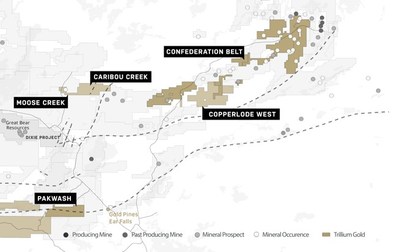
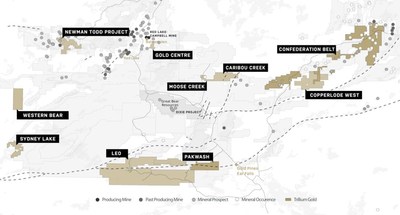
- Award will help to fund planned Phase Ib/IIa study of inhaled murepavadin, a novel class antibiotic for the treatment of chronic Pseudomonas aeruginosa infections in cystic fibrosis
- Development program is currently jointly funded by Polyphor and the European Innovative Medicines Initiative (IMI) until end of planned Phase Ia study
ALLSCHWIL, Switzerland, Nov. 24, 2020 (GLOBE NEWSWIRE) — Polyphor AG (SIX: POLN) today announced a funding agreement with the Cystic Fibrosis Foundation to advance clinical development of its novel class antibiotic, inhaled murepavadin, in cystic fibrosis (CF). Inhaled murepavadin is a highly potent and selective antibiotic against Pseudomonas aeruginosa, including multidrug resistant strains. CF is characterized by chronic bacterial infection and severe inflammation that lead to progressive deterioration in lung function.
Under the terms of the agreement, Polyphor will be awarded up to USD $3.3 million to fund a Phase Ib/IIa clinical trial of inhaled murepavadin, as a follow-up study to a Phase Ia study in healthy volunteers using eFlow® Technology nebulizer (PARI Pharma GmbH), which is planned to be initiated pending CTA (clinical trial application) approval. The Phase Ib/IIa trial in adults with CF, assessing safety and tolerability (both overall and local) of ascending doses of inhaled murepavadin, is planned to be initiated in Q4 2021 following completion of the Phase Ia study. The Cystic Fibrosis Foundation Therapeutics Development Network (TDN) will provide support for the overall clinical development path for inhaled murepavadin.
“Pseudomonas aeruginosa infection is a leading cause of lung function decline in people with cystic fibrosis and our novel class antibiotic, inhaled murepavadin, has the potential to change the treatment paradigm transforming patients’ lives,” said Gokhan Batur, Chief Executive Officer of Polyphor. “This is Polyphor’s second award from the Cystic Fibrosis Foundation, whose support had made possible nearly every cystic fibrosis-specific drug available today. The award will further encourage clinical development of inhaled murepavadin, and we would like to thank the Cystic Fibrosis Foundation for their trust and ongoing support.”
About inhaled murepavadin:
Polyphor’s inhaled murepavadin is currently being developed as a precision antibiotic specifically targeting chronic Pseudomonas aeruginosa, for the treatment of these infections in people with CF. It is the first member of the Outer Membrane Protein Targeting Antibiotics (OMPTA), a novel class of antibiotics which was discovered by Polyphor and the University of Zurich and displays a unique mode of action. Based on the data of the inhaled murepavadin preclinical program suggesting significantly higher safety margins (at least 5-10 times) versus the intravenous formulation, Polyphor is planning to initiate the clinical development program. Until the end of the planned Phase Ia study the program is jointly funded by Polyphor and the European Innovative Medicines Initiative (IMI). Inhaled murepavadin is also part of the iABC project, a Europe-wide program dedicated to the development of inhaled antibiotics run by a consortium of leading lung specialists and research institutions in various European countries. The Cystic Fibrosis Foundation award will allow further development until the end of the Phase Ib/IIa study.
Infections remain a significant problem for people with CF who require novel treatment options, despite the availability of CFTR modulators. If approved for commercial use, inhaled murepavadin would be the first new class of antibiotics for Gram-negative pathogens in the last 50 years. It would also be potentially the first agent to target specifically Pseudomonas aeruginosa bacteria versus the current standard of care, broad spectrum inhaled antibiotics.
For further information please contact:
|
For Investors: Hernan Levett Chief Financial Officer Polyphor Ltd. +41 61 567 16 00 [email protected] |
Mary-Ann Chang LifeSci Advisors Tel: +44 7483 284 853 [email protected] |
For Media:
Bernhard Schmid
LifeSci Advisors
+41 44 447 12 21
[email protected]
About Polyphor
Polyphor is a research-driven clinical-stage, Swiss biopharmaceutical company committed to discovering and developing best-in-class molecules in oncology and antimicrobial resistance leveraging the company’s leading macrocyclic peptide technology platform. Polyphor is advancing balixafortide (POL6326) in a Phase III trial in combination with eribulin in patients with advanced breast cancer and exploring its potential in other cancer indications. In addition, it has discovered and is developing the Outer Membrane Protein Targeting Antibiotics (OMPTA). OMPTA are potentially the first new class of antibiotics in clinical development in the last 50 years against Gram-negative bacteria. The company’s lead OMPTA program is an inhaled formulation of murepavadin for the treatment of Pseudomonas aeruginosa infections in patients with cystic fibrosis. Polyphor is based in Allschwil near Basel and is listed on the SIX Swiss Exchange (SIX: POLN). For more information, please visit www.polyphor.com.
Disclaimer
This press release contains forward-looking statements which are based on current assumptions and forecasts of the Polyphor management. Known and unknown risks, uncertainties, and other factors could lead to material differences between the forward-looking statements made here and the actual development, in particular Polyphor’s results, financial situation, and performance. Readers are cautioned not to put undue reliance on forward-looking statements, which speak only of the date of this communication. Polyphor disclaims any intention or obligation to update and revise any forward-looking statements, whether as a result of new information, future events or otherwise.