ADJUSTED ATTRIBUTABLE EPS OF $0.49 PER SHARE
DENVER, Nov. 12, 2020 /PRNewswire/ – SSR Mining Inc. (NASDAQ: SSRM) (TSX: SSRM) (ASX: SSR) (“SSR Mining”) reports consolidated financial results for the third quarter ended September 30, 2020.
Rod Antal, President and CEO said, “With the transformational merger with Alacer Gold finalized, integration efforts near completion, and our operations running at steady state following COVID-19 interruptions, the focus has turned towards delivering a number of value enhancing catalysts before year-end.
From a growth perspective, we intend on publishing the Çöpler technical report and global exploration updates in the coming weeks. Operationally, we anticipate a robust fourth quarter with strong free cash flow generation, further strengthening our balance sheet. This continued peer-leading free cash flow generation has allowed us to put in place a dividend policy beginning in the first quarter of 2021. Our capital allocation strategy is intended to balance investment in high-return growth opportunities, maintaining peer leading financial strength, and providing sustainable capital returns to shareholders. A recurring quarterly dividend is expected to be the primary method of capital return, and we will periodically evaluate supplementing this dividend from trailing excess attributable free cash flow through incremental dividends and/or share buyback programs.”
Third Quarter 2020 Highlights:
(All figures are in U.S. dollars unless otherwise noted)
-
Closed zero-premium merger with Alacer: Completed the transaction with Alacer to create a leading intermediate precious metals producer with robust margins, strong free cash flow generation and long mine lives led by a highly experienced leadership team with a track record of value creation.
-
Dividend policy announced: On November 12, 2020, the Board approved the initiation of a quarterly cash dividend of $0.05 per share beginning in the first quarter of 2021. In addition, the Board will periodically consider supplementing the quarterly dividend from trailing excess attributable free cash flow in the form of incremental dividends and/or share buyback programs.
-
On-track to meet full year 2020 updated production guidance: Year-to-date production of 491,821 gold equivalent ounces across the four operations.(1)
-
Updated full year 2020 outlook on completion of merger: Production of 680,000 to 760,000 gold equivalent ounces at AISC of $965 to $1,040 per gold equivalent ounce.(2)
-
Strong financial performance: Reported attributable net income of $26.8 million, or $0.19 per share and adjusted attributable net income of $67.8 million, or $0.49 per share.(2) At the beginning of the fourth quarter all four mines were at steady-state operation.
-
Maintained strong balance sheet and liquidity: Consolidated cash (2) balance at quarter end increased to $772.8 million, further strengthening our balance sheet.
-
Çöpler contributes low-cost production: From the date of acquisition to quarter-end, produced 19,586 ounces of gold at AISC of $737 per ounce.(2) Produced 76,666 ounces of gold during the full quarter. Pit cutback to provide access to higher grade ore expected in the fourth quarter of 2020.
-
Strong operational result at Marigold: Produced 49,137 ounces of gold at AISC of $1,243 per ounce.(2) Stacked over 73,000 recoverable ounces of gold in the third quarter, setting the operation up for a strong finish to the year.
-
Safe ramp-up of operations at Seabee: Produced 20,249 ounces of gold at AISC of $988 per ounce.(2) Mill throughput averaged 1,271 tonnes per day in September 2020, a monthly record.
-
Strong margins at Puna: Produced 1.3 million ounces of silver at AISC of $11.26 per ounce.(2) Generated income from mine operations of $17.4 million in the third quarter.
-
Continued exploration success: Encouraging exploration results in the third quarter across our portfolio at Çöpler, Marigold and Seabee.
|
(1)
|
Year-to-date production includes full year 2020 production from Çöpler. SSR Mining is not entitled to the economic benefits from Çöpler prior to acquisition.
|
|
(2)
|
We report the non-GAAP financial measures of all-in sustaining costs (“AISC”) per ounce of gold and silver sold, adjusted attributable net income, adjusted attributable net income per share and consolidated cash to manage and evaluate our operating performance. Note that we have made adjustments to the items which are included in the determination of adjusted attributable net income compared to prior periods. See “Cautionary Note Regarding Non-GAAP Measures”.
|
Cöpler, Turkey
(amounts presented on 100% basis)
|
|
Period from acquisition
to September 30
|
Three months ended
September 30
|
Nine months ended
September 30
|
|
Operating Data
|
2020 (1)
|
2020 (2)
|
2020 (2)
|
|
Ore mined – oxide (kt)
|
147
|
497
|
1,469
|
|
Ore mined – sulfide (kt)
|
99
|
715
|
1,320
|
|
Total material mined (kt)
|
1,063
|
6,851
|
18,816
|
|
Waste removed (kt)
|
817
|
5,639
|
16,027
|
|
Strip ratio
|
3.3
|
4.7
|
5.7
|
|
|
|
|
|
|
Ore stacked – oxide (kt)
|
121
|
535
|
1,549
|
|
Gold grade stacked – oxide (g/t)
|
1.24
|
0.97
|
1.08
|
|
|
|
|
|
|
Ore processed – sulfide (kt)
|
85
|
530
|
1,573
|
|
Gold grade processed – sulfide (g/t)
|
3.74
|
3.65
|
3.79
|
|
Gold recovery – sulfide (%)
|
89.0
|
90.7
|
90.8
|
|
|
|
|
|
|
Gold produced – oxide (oz)
|
5,258
|
19,617
|
66,348
|
|
Gold produced – sulfide (oz)
|
14,328
|
57,049
|
177,530
|
|
Total gold produced (oz)
|
19,586
|
76,666
|
243,878
|
|
Gold sold (oz)
|
27,895
|
74,665
|
240,885
|
|
|
|
|
|
|
Average realized gold price ($/oz sold)
|
$
|
1,920
|
$
|
1,902
|
$
|
1,725
|
|
|
|
|
|
|
Cash costs ($/oz gold sold) (3, 4)
|
$
|
609
|
N/A
|
N/A
|
|
AISC ($/oz gold sold) (3, 4)
|
$
|
737
|
N/A
|
N/A
|
|
|
|
|
|
|
Financial Data ($000s)
|
|
|
|
|
Revenue
|
$
|
53,566
|
N/A
|
N/A
|
|
Production costs
|
$
|
40,670
|
N/A
|
N/A
|
|
Depletion and depreciation
|
$
|
8,895
|
N/A
|
N/A
|
|
Income from mine operations
|
$
|
4,001
|
N/A
|
N/A
|
|
Exploration and evaluation expenses
|
$
|
953
|
N/A
|
N/A
|
|
Capital expenditures
|
$
|
4,420
|
N/A
|
N/A
|
|
(1)
|
The data presented in this column is for the period from September 16, 2020 to September 30, 2020, the period for which we were entitled to all economic benefits of Çöpler following our acquisition of Alacer.
|
|
(2)
|
The operating data presented in these columns includes operating results for Çöpler for the entire three and nine months ended September 30, 2020, including the period prior to our acquisition of Alacer on September 16, 2020. As we were not entitled to the economic benefits of Çöpler prior to the acquisition, financial results for the period prior to September 16, 2020 are not provided.
|
|
(3)
|
We report the non-GAAP financial measures of cash costs and AISC per ounce of gold sold to manage and evaluate operating performance at Çöpler. For further information, please refer to “Cautionary Note Regarding Non-GAAP Measures”.
|
|
(4)
|
Cash costs and AISC per ounce of gold sold exclude the impact of the write-up of inventory to fair value as at the date of our acquisition of Alacer.
|
Production
Çöpler produced 19,586 ounces of gold from September 16, 2020, the date of our acquisition of Alacer, to the end of the third quarter of 2020 (the “Period”).
Gold production from the Çöpler oxide plant was 19,617 and 66,348 ounces for the three and nine months ended September 30, 2020, respectively. Gold production from the sulfide plant was 57,049 and 177,530 ounces for the three and nine months ended September 30, 2020, respectively.
Çöpler produced 76,666 and 243,878 ounces of gold for the three and nine months ended September 30, 2020, respectively. Production was in line with the revised mine plan that was adopted in the second quarter of 2020 to diversify ore sources and optimize production in light of the shortfall in mine operators resulting from COVID-19.
Oxide ore tonnes mined in the three and nine months ended September 30, 2020 were 0.5 million and 1.5 million tonnes, respectively. The contained gold ounces in the oxide ore mined were 11,575 and 45,334 for the three and nine months ended September 30, 2020, respectively, lower than similar periods in the prior year, but in line with the mine plan, due to the completion of Çakmaktepe phase one mining in 2019. The oxide ore mined grade was 0.72 g/t and 0.96 g/t for the three and nine months ended September 30, 2020, respectively.
The stacked oxide ore contained 16,747 and 53,563 ounces of gold for the three and nine months ended September 30, 2020, respectively.
Sulfide ore tonnes mined in the three and nine months ended September 30, 2020 were 0.7 million and 1.3 million tonnes, respectively, in line with the revised mine plan referred to above.
The sulfide plant treated 0.5 million and 1.6 million tonnes of sulfide ore for the three and nine months ended September 30, 2020, respectively. The sulfide plant continued to efficiently operate above design throughput, though marginally below that of the prior quarter as autoclave 1 was shutdown in July 2020 to perform inspections, which indicated it was in excellent condition. Plant gold recovery has averaged approximately 91% through September 30, 2020. Projects are underway to increase gold recovery. Some improvement in recovery was achieved by installation of oxygen injection into the leach tanks which was commissioned in late June.
Mine operator availability suffered in 2020 due to COVID-19 restrictions. The revised plan reduced the over-haulage of extra material to the tailings storage facility (“TSF”), allowing for available mining resources to be focused on the Manganese pit cutback. The TSF is an approximately 5 km haul from the mine and is constructed from competent mine waste. Despite the reduced construction rate this year, the TSF is advancing ahead of operational requirements. The Manganese pit cutback remains on track to provide access to higher grade ore in the fourth quarter of 2020. A mining area was also brought into production in the Main pit to diversify ore sources, in part, as a risk management strategy should COVID-19 related restrictions increase.
The total waste tonnes mined in the three and nine months ended September 30, 2020 were 5.6 million and 16.0 million tonnes, respectively, in line with the revised mine plan.
The 2020 Çöpler technical report, planned to be issued in the fourth quarter of 2020, will update the operating parameters of the plant and include the results of optimization studies and programs, including the proposed supplemental flotation circuit. Detailed engineering for the proposed flotation circuit is underway and the final construction decision remains subject to final Board and other approvals once the technical work is complete. If approved, the flotation circuit commissioning is targeted for the third quarter of 2021. The proposed flotation circuit was included in the recent amendment to the Çöpler Environmental Impact Assessment application.
The proposed flotation circuit would treat a side stream from the grinding circuit, with the concentrate reporting to autoclave feed and the tails to leaching. Studies indicate a negative recovery impact due to the float tails recovery; however, such impact will be more than offset by increased plant throughput. The currently-installed grinding mills have demonstrated significant latent capacity, sufficient to support an increase in sulfide plant throughput capacity up to approximately 3 million tonnes per annum. The preliminary capital estimate for the proposed flotation circuit is approximately $15 million. The flotation circuit is anticipated to increase the gold and sulfur grades processed through the autoclaves (increasing autoclave and oxygen utilization), reduce unit costs, and increase total sulfide plant throughput and gold production.
Revenue
Revenue for the Period was $53.6 million as 27,895 ounces of gold were sold at an average realized gold price of $1,920 per ounce. Finished goods inventory that was built-up prior to acquisition was sold during the Period.
Operating Costs
Cash costs and AISC per ounce of gold sold are non-GAAP financial measures. Please see the discussion under “Cautionary Note Regarding Non-GAAP Measures”.
Unit operating costs remained stable as a weaker local currency helped offset COVID-19 associated impacts to operating efficiencies. Cash costs per ounce sold in the Period were $609. Cash costs were impacted by a higher royalty expense due to higher realized gold prices.
AISC per ounce sold in the Period was $737 which is lower than planned due to lower sustaining capital spend, primarily related to the deferral in TSF expansion as discussed above and delays in Sabirli Road and heap leach expansion construction due to COVID-19 related manning shortfalls.
Marigold, USA
|
|
Three months ended September 30
|
Nine months ended September 30
|
|
|
|
|
|
Operating Data
|
2020
|
2019
|
Change
|
2020
|
2019
|
Change
|
|
Total material mined (kt)
|
20,582
|
19,033
|
8%
|
62,895
|
55,583
|
13%
|
|
Waste removed (kt)
|
13,890
|
12,676
|
10%
|
46,092
|
36,628
|
26%
|
|
Total ore stacked (kt)
|
6,692
|
6,357
|
5%
|
16,803
|
18,955
|
(11)%
|
|
Gold stacked grade (g/t)
|
0.43
|
0.51
|
(16)%
|
0.35
|
0.41
|
(15)%
|
|
Strip ratio
|
2.1
|
2.0
|
5%
|
2.7
|
1.9
|
42%
|
|
|
|
|
|
|
|
|
|
Gold produced (oz)
|
49,137
|
52,968
|
(7)%
|
157,502
|
161,041
|
(2)%
|
|
Gold sold (oz)
|
51,700
|
50,650
|
2%
|
156,117
|
165,871
|
(6)%
|
|
|
|
|
|
|
|
|
|
Average realized gold price
($/oz sold)
|
$
|
1,912
|
$
|
1,481
|
29%
|
$
|
1,735
|
$
|
1,359
|
28%
|
|
|
|
|
|
|
|
|
|
Cash costs ($/oz gold sold) (1)
|
$
|
899
|
$
|
822
|
9%
|
$
|
861
|
$
|
823
|
5%
|
|
AISC ($/oz gold sold) (1)
|
$
|
1,243
|
$
|
1,104
|
13%
|
$
|
1,297
|
$
|
1,003
|
29%
|
|
|
|
|
|
|
|
|
|
Financial Data ($000s)
|
|
|
|
|
|
|
|
Revenue
|
$
|
98,748
|
$
|
74,820
|
32%
|
$
|
270,615
|
$
|
225,122
|
20%
|
|
Production costs
|
$
|
46,387
|
$
|
41,551
|
12%
|
$
|
134,181
|
$
|
136,310
|
(2)%
|
|
Depletion and depreciation
|
$
|
10,737
|
$
|
11,205
|
(4)%
|
$
|
32,092
|
$
|
39,828
|
(19)%
|
|
Income from mine operations
|
$
|
41,624
|
$
|
22,064
|
89%
|
$
|
104,342
|
$
|
48,984
|
113%
|
|
Exploration and evaluation expenses
|
$
|
953
|
$
|
893
|
7%
|
$
|
2,035
|
$
|
1,380
|
47%
|
|
Capital expenditures
|
$
|
16,532
|
$
|
13,256
|
25%
|
$
|
64,329
|
$
|
27,778
|
132%
|
|
(1)
|
We report the non-GAAP financial measures of cash costs and AISC per ounce of gold sold to manage and evaluate operating performance at Marigold. For further information, please refer to “Cautionary Note Regarding Non-GAAP Measures”.
|
Production
In the third quarter of 2020, 20.6 million tonnes of material were mined, an 8% increase compared to the third quarter of 2019, reflecting the impact of shorter haulage cycles coupled with increased shovel fleet capacity. For the nine months ended September 30, 2020, 62.9 million tonnes of material were mined, a 13% increase over the first nine months of 2019. The increase is attributable to shorter haulage cycles, the addition of one haul truck to the fleet and increases in shovel fleet capacity.
During the third quarter of 2020, 6.7 million tonnes of ore was stacked at a gold grade of 0.43 g/t. This compares to 6.4 million tonnes of ore was stacked at a gold grade of 0.51 g/t in the third quarter of 2019. Higher tonnes stacked at a lower grade are associated with the transition of primary mining from the higher grade lower levels of Mackay 5 in the third quarter of 2019 to the upper levels of Mackay 4, which transitioned from stripping to primary ore delivery in the third quarter of 2020.
For the nine months ended September 30, 2020, 16.8 million tonnes of ore was stacked at a gold grade of 0.35 g/t compared to approximately 19.0 million tonnes of ore stacked at a gold grade of 0.41 g/t for the first nine months of 2019. The reduction in both ore tonnes stacked and gold grade are in line with the mine plan and associated with the transition from mining Mackay 5 ore mining in 2019 to the stripping and mining of Mackay Phases 4 and 8 in 2020.
During the third quarter of 2020, Marigold produced 49,137 ounces of gold, a decrease of 7% compared to the third quarter of 2019, predominantly due to lower gold grades stacked within the previous three months. Recoverable ounces stacked in the third quarter of 2020 and 2019 were 73,595 and 79,306, respectively. The majority of recoverable ounces stacked in the third quarter of 2020 were at low elevations on the heap leach pads to facilitate faster leach recovery times.
For the nine months ended September 30, 2020, 157,502 ounces of gold were produced compared to 161,041 ounces of gold over the same period in 2019.
Revenue
Revenue increased by 32% to $98.7 million in the third quarter of 2020 as compared to the third quarter of 2019 due to an increase of 29% in the average realized gold price as well as 2% more ounces sold.
Revenue increased 20% for the nine months ending September 30, 2020 as compared to the same period in the prior year, due to an increase of 28% in the average realized gold price slightly offset by 6% fewer ounces sold.
Operating Costs
Cash costs and AISC per ounce of gold sold are non-GAAP financial measures. Please see the discussion under “Cautionary Note Regarding Non-GAAP Measures”.
In the third quarter of 2020, cash costs per ounce of gold sold were $899, a 9% increase compared to the third quarter of 2019, primarily due to an increase in per unit royalty costs due to higher realized gold prices and the impact of lower grades mined which has resulted in a higher cost per ounce in inventory.
In the third quarter of 2020, AISC per ounce of gold sold was $1,243, a 13% increase compared to the third quarter of 2019, due to higher cash costs and an increase in capital expenditures per gold ounce sold. Capital expenditures were higher than in the third quarter of 2019 primarily due to mine equipment purchases as well as increased leach pad and dewatering construction costs. Mine equipment additions included two replacement haul trucks and support equipment.
Cash costs per ounce of gold sold for the nine months ending September 30, 2020 were $861, a 5% increase compared to the same period of 2019, primarily due to an increase in per unit royalty costs due to higher realized gold prices.
AISC per ounce of gold sold for the nine months ending September 30, 2020 was $1,297, a 29% increase compared to the same period of 2019 due to higher cash costs and an increase in capital expenditures per gold ounce sold. Capital expenditures were higher than the nine months ending September 30, 2019, primarily due to new mobile mine equipment purchases, as well as increased leach pad and dewatering construction costs.
Seabee, Canada
|
|
Three months ended September 30
|
Nine months ended September 30
|
|
|
|
|
|
Operating Data
|
2020
|
2019
|
Change
|
2020
|
2019
|
Change
|
|
Total ore milled (t)
|
66,409
|
77,465
|
(14)%
|
155,690
|
256,645
|
(39)%
|
|
Ore milled per day (t/day)
|
722
|
842
|
(14)%
|
568
|
940
|
(40)%
|
|
Gold mill feed grade (g/t)
|
10.17
|
12.39
|
(18)%
|
10.27
|
10.16
|
1%
|
|
Gold recovery (%)
|
98.6
|
98.8
|
—
|
98.3
|
98.2
|
—
|
|
|
|
|
|
|
|
|
|
Gold produced (oz)
|
20,249
|
32,345
|
(37)%
|
49,770
|
90,068
|
(45)%
|
|
Gold sold (oz)
|
19,900
|
28,278
|
(30)%
|
47,614
|
80,553
|
(41)%
|
|
|
|
|
|
|
|
|
|
Average realized gold price
($/oz sold)
|
$
|
1,913
|
$
|
1,480
|
29%
|
$
|
1,739
|
$
|
1,372
|
27%
|
|
|
|
|
|
|
|
|
|
Cash costs ($/oz sold) (1)
|
$
|
538
|
$
|
373
|
44%
|
$
|
542
|
$
|
452
|
20%
|
|
AISC ($/oz sold) (1)
|
$
|
988
|
$
|
715
|
38%
|
$
|
1,035
|
$
|
830
|
25%
|
|
|
|
|
|
|
|
|
|
Financial Data ($000s)
|
|
|
|
|
|
|
|
Revenue
|
$
|
38,035
|
$
|
41,331
|
(8)%
|
$
|
82,732
|
$
|
109,999
|
(25)%
|
|
Production costs
|
$
|
10,677
|
$
|
10,426
|
2%
|
$
|
25,725
|
$
|
36,187
|
(29)%
|
|
Depletion and depreciation
|
$
|
7,167
|
$
|
8,771
|
(18)%
|
$
|
17,085
|
$
|
26,244
|
(35)%
|
|
Income from mine operations
|
$
|
20,191
|
$
|
22,134
|
(9)%
|
$
|
39,922
|
$
|
47,568
|
(16)%
|
|
Exploration and evaluation expenses
|
$
|
1,108
|
$
|
2,131
|
(48)%
|
$
|
4,020
|
$
|
7,300
|
(45)%
|
|
Capital expenditures
|
$
|
10,371
|
$
|
8,759
|
18%
|
$
|
25,555
|
$
|
27,613
|
(7)%
|
|
(1)
|
We report the non-GAAP financial measures of cash costs and AISC per ounce of gold sold to manage and evaluate operating performance at Seabee. For further information, please refer to “Cautionary Note Regarding Non-GAAP Measures”.
|
Production
In response to the COVID-19 pandemic, Seabee was voluntarily placed into temporary care and maintenance on March 25, 2020 as a precautionary measure to protect our employees, their families and our communities. Through this period, employees maintained the mine in a state of operational readiness.
In June 2020, a phased restart of the operation commenced. The first phase focused on underground ventilation raises and capital development within the mine while COVID-19-related protocols were assessed. Limited ore extraction was initiated at the end of June. In early July, we commenced the second phase, which involved increasing underground development rates and mine production while continuing to monitor COVID-19 related protocols. In August, the third and final phase commenced, which involved a restart of milling operations and ramp-up to full mine production with a complete workforce, while continuing to maintain effective COVID-19-related protocols. The mine has operated at full capacity since that date.
For the three months ended September 30, 2020, Seabee produced 20,249 ounces of gold, a 37% decrease compared to the same period in the prior year, reflecting that the mill restarted operations in early August 2020. Mill feed grade was 10.17 g/t gold during the three months ended September 30, 2020, an 18% decrease compared to the same period in the prior year, due to the natural sequencing of the mine plan. Mill throughput achieved a monthly record 1,271 tonnes per day in September 2020 and ore stockpile at the end of the third quarter totaled over 17,000 tonnes.
For the nine months ended September 30, 2020, Seabee produced 49,770 ounces of gold, a 45% decrease compared to the same period in the prior year, reflecting that the mill shut and restarted operations in March and August 2020, respectively, whereas the mill was fully operational in 2019.
Revenue
Revenue decreased by 8% in the third quarter of 2020 as compared to the same period in the prior year, due to a 30% decrease in gold ounces sold compared to the third quarter of 2019, largely offset by an increase of 29% in the average realized gold price. The decrease in sales volume was caused by lower production associated with the temporary suspension of milling operations during the beginning of the third quarter of 2020 in response to the COVID-19 pandemic.
Revenue decreased by 25% for the nine months ended September 30, 2020 as compared to the same period in the prior year, due to a 41% decrease in gold ounces sold compared to the same period of 2019, partially offset by an increase of 27% in the average realized gold price. The decrease in sales volume was caused by lower production associated with the temporary suspension of milling operations for all of the second quarter and beginning of the third quarter of 2020 in response to the COVID-19 pandemic.
Operating Costs
Cash costs and AISC per ounce of gold sold are non-GAAP financial measures. Please see the discussion under “Cautionary Note Regarding Non-GAAP Measures”.
In the third quarter of 2020, cash costs per ounce of gold sold were $538, a 44% increase compared to the third quarter of 2019. Third quarter 2019 cash costs were positively impacted by ounces recovered from carbon and certain cost reclassifications. The increase is also due to the impact of lower gold sales volume, coupled with incremental costs associated with the COVID-19 pandemic and fixed general and administrative expenses associated with ramp up to full production. Expenditures incurred while Seabee’s operations were temporarily suspended were classified as care and maintenance expenses.
In the third quarter of 2020, AISC per ounce of gold sold was $988, a 38% increase compared to the third quarter of 2019, due to higher cash costs and an increase in capital expenditures per gold ounce sold due to lower ounces sold in the quarter. Capital expenditures related mainly to an increase in underground capital development completed during the temporary shutdown and the tailings expansion project. The tailings expansion project contractor resumed full construction activities in early August 2020.
For the nine months ended September 30, 2020, cash costs per ounce of gold sold were $542, a 20% increase compared to the same period in the prior year, due to higher unit mining and general and administrative costs, driven by the temporary suspension of operations for all of the second quarter and beginning of the third quarter, as well as incremental costs associated with the COVID-19 pandemic.
For the nine months ended September 30, 2020, AISC per ounce of gold sold was $1,035, a 25% increase, due to higher cash costs and an increase in capital expenditures per gold ounce sold. Capital expenditures incurred to date in 2020 are in-line with budget.
Puna, Argentina
(amounts presented on 100% basis)
|
|
Three months ended September 30
|
Nine months ended September 30
|
|
|
|
|
|
Operating Data
|
2020
|
2019
|
Change
|
2020
|
2019
|
Change
|
|
Total material mined (kt)
|
902
|
3,116
|
(71)%
|
2,945
|
9,024
|
(67)%
|
|
Waste removed (kt)
|
722
|
2,531
|
(71)%
|
2,439
|
8,099
|
(70)%
|
|
Strip ratio
|
4.0
|
4.3
|
(7)%
|
4.8
|
8.8
|
(45)%
|
|
|
|
|
|
|
|
|
|
Ore milled (kt)
|
284
|
336
|
(15)%
|
703
|
933
|
(25)%
|
|
Silver mill feed grade (g/t)
|
150
|
165
|
(9)%
|
154
|
188
|
(18)%
|
|
Lead mill feed grade (%)
|
0.71
|
0.81
|
(12)%
|
0.77
|
0.87
|
(11)%
|
|
Zinc mill feed grade (%)
|
0.57
|
0.60
|
(5)%
|
0.51
|
0.51
|
—
|
|
Silver recovery (%)
|
93.5
|
93.5
|
—
|
94.2
|
92.4
|
2%
|
|
Lead recovery (%)
|
88.3
|
88.1
|
—
|
89.8
|
84.0
|
7%
|
|
Zinc recovery (%)
|
52.4
|
49.3
|
6%
|
51.2
|
48.3
|
6%
|
|
|
|
|
|
|
|
|
|
Silver produced (‘000 oz)
|
1,280
|
1,664
|
(23)%
|
3,416
|
5,541
|
(38)%
|
|
Silver sold (‘000 oz)
|
1,193
|
1,505
|
(21)%
|
3,651
|
5,111
|
(29)%
|
|
Lead produced (‘000 lb) (1)
|
3,952
|
5,304
|
(25)%
|
10,664
|
15,972
|
(33)%
|
|
Lead sold (‘000 lb) (1)
|
3,655
|
4,119
|
(11)%
|
11,745
|
14,748
|
(20)%
|
|
Zinc produced (‘000 lb) (1)
|
1,876
|
2,206
|
(15)%
|
4,056
|
5,385
|
(25)%
|
|
Zinc sold (‘000 lb) (1)
|
1,557
|
2,030
|
(23)%
|
4,141
|
11,005
|
(62)%
|
|
|
|
|
|
|
|
|
|
Average realized silver price ($/oz)
|
$
|
26.69
|
$
|
17.31
|
54%
|
$
|
20.25
|
$
|
15.71
|
29%
|
|
|
|
|
|
|
|
|
|
Cash costs ($/oz silver sold) (2,3)
|
$
|
9.33
|
$
|
14.22
|
(34)%
|
$
|
12.13
|
$
|
11.15
|
9%
|
|
AISC ($/oz silver sold) (2,3)
|
$
|
11.26
|
$
|
17.36
|
(35)%
|
$
|
15.03
|
$
|
15.55
|
(3)%
|
|
|
|
|
|
|
|
|
|
Financial Data ($000s)
|
|
|
|
|
|
|
|
Revenue
|
$
|
35,063
|
$
|
31,697
|
11%
|
$
|
75,447
|
$
|
94,126
|
(20)%
|
|
Production costs
|
$
|
13,112
|
$
|
22,638
|
(42)%
|
$
|
48,495
|
$
|
68,134
|
(29)%
|
|
Depreciation and depletion
|
$
|
4,541
|
$
|
1,351
|
236%
|
$
|
13,031
|
$
|
10,574
|
23%
|
|
Income from mine operations
|
$
|
17,410
|
$
|
7,708
|
126%
|
$
|
13,921
|
$
|
15,418
|
(10)%
|
|
Exploration and evaluation expense
|
$
|
38
|
$
|
230
|
(83)%
|
$
|
193
|
$
|
295
|
(35)%
|
|
Capital expenditures
|
$
|
4,616
|
$
|
4,857
|
(5)%
|
$
|
11,210
|
$
|
28,262
|
(60)%
|
|
(1)
|
Data for lead production and sales relate only to lead in lead concentrate. Data for zinc production and sales relate only to zinc in zinc concentrate.
|
|
(2)
|
We report the non-GAAP financial measures of cash costs and AISC per ounce of silver sold to manage and evaluate operating performance at Puna. For further information, please refer to “Cautionary Note Regarding Non-GAAP Measures”.
|
|
(3)
|
Puna cash costs and AISC per silver ounce sold include a write-down of metal inventories to net realizable value of nil and $8.6 million for the three and nine months ended September 30, 2020, respectively (three and nine months ended September 30, 2019 – $1.8 million and $2.4 million, respectively).
|
Production
On March 20, 2020, Puna temporarily suspended operations as a result of government-mandated restrictions due to the COVID-19 pandemic. Subsequently, the Government of Argentina reinstated mining as an essential business activity. During the second quarter of 2020, a phased restart complying with government regulations and guidelines was implemented with mining, hauling and milling operations re-commencing. During the third quarter of 2020, COVID-19 infection rates in the province of Jujuy escalated, resulting in further interruptions to operations. In September, operations were suspended in order to manage camp occupancy, conduct testing and reduce the risk of transmission.
Due to the significant ore stockpiles at Puna, milling operations were prioritized over mining operations through restarts. As a result, tonnes mined in the third quarter of 2020 were impacted due to COVID-19 related interruptions. Mining and milling activities were operating at expected levels by the beginning of October.
In the third quarter of 2020, Puna produced 1.3 million ounces of silver, a 23% decrease compared to the third quarter of 2019, due to the temporary suspension of operations during September in response to COVID-19. Ore milled was 0.3 million tonnes, a 15% decrease compared to the third quarter of 2019 as a result of fewer operating days. Processed ore contained an average silver grade of 150 g/t, a 9% decrease compared to the third quarter of 2019, but in-line with the mine plan. When operational, the mill averaged approximately 4,247 tonnes per day during the third quarter of 2020, demonstrating the improved performance of the plant and tailings pumping system.
For the nine months ended September 30, 2020, Puna produced 3.4 million ounces of silver, a 38% decrease compared to the same period in the prior year, due to the temporary suspension of operations in response to COVID-19 during most of the second quarter and part of the third quarter of 2020. Ore milled was 0.7 million tonnes, a 25% decrease compared to the nine months ended September 30, 2019, as a result of fewer operating days. Processed ore contained an average silver grade of 154 g/t, an 18% decrease compared to the same period in the prior year, but in-line with the mine plan.
Revenue
Revenue for the third quarter of 2020 increased by 11% compared to the third quarter of 2019, due to a 54% increase in the average realized silver price in the third quarter of 2020, partially offset by a 21% decrease in silver ounces sold.
Revenue for the nine months ended September 30, 2020 decreased by 20% compared to the same period in the prior year, due to a 29% decrease in silver ounces sold, partially offset by a 29% increase in the average realized silver price.
Operating Costs
Cash costs and AISC per ounce of silver sold are non-GAAP financial measures. Please see the discussion under “Cautionary Note Regarding Non-GAAP Measures”.
In the third quarter of 2020, cash costs per ounce of silver sold were $9.33, a decrease of 34% compared to the third quarter of 2019, primarily due to lower unit processing and general and administrative costs as a result of higher average daily plant throughput. Expenditures incurred during the quarter that were not related to operating activities were classified as care and maintenance expenses.
In the third quarter of 2020, AISC per ounce of silver sold was $11.26, a decrease of 35% compared to the third quarter of 2019. The decrease in AISC was primarily due to lower cash costs for the period.
For the nine months ended September 30, 2020, cash costs per ounce of silver sold were $12.13, an increase of 9% compared to the same period in the prior year. The increase is primarily due to higher mining unit costs, offset by lower processing and general and administrative unit costs as a result of higher average daily plant throughput. Mining costs were higher due to operating inefficiencies through shut-down and start-up phases and an increase in maintenance work performed during the temporary suspensions.
For the nine months ended September 30, 2020, AISC per ounce of silver sold was $15.03, a decrease of 3% compared to the same period on the prior year, due to 34% lower capital expenditures per ounce sold, mainly due to lower deferred stripping costs, offset partially by higher cash costs.
Exploration and Development
We hold a portfolio of prospective exploration tenures across Turkey, the USA, Canada, Mexico and Peru, some of which are becoming advanced projects. We continue to explore and expand our development pipeline, looking for both near-mine projects that can leverage existing mine infrastructure and new standalone projects.
Çöpler District Exploration
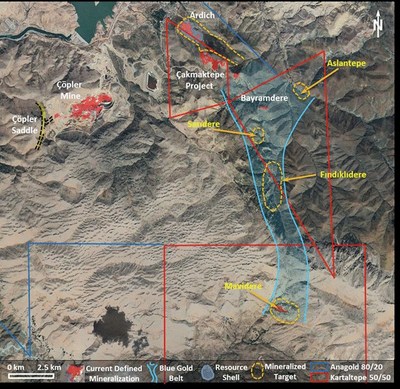
We take a disciplined approach to exploration at our Çöpler District and elsewhere, optimizing the historical exploration database, remapping and reinterpreting data, and judicious drill testing new targets. Recent discoveries, such as the Saddle prospect, Çakmaktepe, the Ardich deposit and other advanced exploration targets, prove the effectiveness of our systemic and pragmatic approach.
Our capital allocation focus is to fast-track the extension of Çöpler oxide ore production, along with the mobilization of a project and development team to deliver the growth potential identified around the Çöpler District.
The Saddle prospect, Çakmaktepe and the Ardich deposit represent the next phase of priority as near–mine development projects with potential to add near-surface ounces to our production profile within the next two to three years. The current work program includes definition drilling to understand the Mineral Resource distribution and provide samples for metallurgical characterization. For project development, work is underway to optimize pit designs and advance permitting.
Çöpler (80% owned by SSR Mining)
The operating mine is the foundation for district exploration activities, with established infrastructure for treating both oxide and sulfide gold ores.
Commencing in 2017, a Çöpler in–pit exploration program successfully provided additional oxide ore to the processing facilities. The in–pit exploration program is ongoing, targeting both oxide and sulfide ore, with some contribution being included in the 2020 production schedules. Recently, the in-pit exploration program identified the possibility of a copper-gold porphyry system below the Main pit. Drill testing of this target commenced at the end of the second quarter of 2020 and continued through the third quarter. Initial drill results are encouraging.
The potential for heap leach pad constraints has been eliminated with the progression of an approximate 25 million tonne Çöpler heap leach pad expansion, that will be built in phases over the coming years as required for the mine plan.
Çöpler Saddle (80% owned by SSR Mining)
The Saddle prospect borders the western flank of Çöpler as a two km long north-south shear zone passing through West pit. The Company announced assays for 50 drill holes at the Saddle prospect in September 2019, which relate to mineralization outside current Mineral Resources.
Çakmaktepe Mine (50% owned by SSR Mining)
Connected by a haul road, Çakmaktepe lies five km east of our processing infrastructure. In 2019, Phase 1 was mined. Exploration is focused on the connection to Ardich which is immediately adjacent to the northeast of Çakmaktepe.
Ardich Gold Deposit (80% owned by SSR Mining)
The Ardich gold deposit is six km northeast of the Çöpler processing facilities and is accessible by the nearby haul road to Çakmaktepe. The deposit mostly forms a tabular flat-lying gold-rich oxide and sulfide zone at the contact between an overlying assemblage of ultramafic rocks and underlying clastic and limestone rock types. The deposit is predominantly oxide mineralization.
During the third quarter of 2020, activities included both step-out and infill drilling to increase confidence in the Mineral Resource and provide data on metallurgical and geotechnical characteristics. The 2020 Çöpler technical report, planned to be issued in the fourth quarter of 2020, will include a preliminary economic assessment for a starter pit option for Ardich. Drilling continues at Ardich as the resource remains open.
The Mavialtin Porphyry Belt (50% owned by SSR Mining)
The Mavialtin Porphyry Belt represents at least four gold-copper porphyry type exploration targets over a seven by 20 km area from Çakmaktepe in the north to the deposit at Mavidere in the south. In February 2020, positive drill results were announced for Mavidere, Findiklidere, and Aslantepe. The mineralization is close to surface and appears to be low in deleterious elements.
The exploration and future development strategy for Mavialtin is two–fold:
- Expand the known areas of mineralization, while concurrently making new discoveries, to economically justify a stand-alone mine; and/or
- Develop a Mavialtin Complex where various smaller deposits could be processed through a central facility.
Mavialtin’s future developmental potential and optionality are illustrated by:
- Proximity to existing operations/infrastructure in the Çöpler Gold Mining District;
- Near-surface nature of the mineralization;
- Length of the mineralized intercepts which indicate the potential for volume; and
- Some high-grade intercepts
Drill testing of Findiklidere and Saridere continued through the third quarter of 2020.
Demirmağara Prospect (80% owned by SSR Mining)
The Demirmağara prospect has both epithermal mineralization and evidence of porphyry alteration with areas of elevated soil and rock, gold and copper geochemistry.
Similar to the other prospects in the portfolio, our exploration team has taken a very disciplined approach to exploration in Demirmağara, such as data mining our historical exploration database, remapping, reinterpretation and conservative drill confirmation of models, resulting in a re-interpretation of Demirmağara. Subsequently, we discovered a covered porphyry stockwork system near surface which was identified by trench sampling revealing potassic granodiorites. We have received forestry permits and plans to drill test the copper gold porphyry target.
Copper Hill Copper Exploration Prospect (50% owned by SSR Mining)
In April 2020, encouraging drill results from the Copper Hill exploration prospect in the Black Sea region (northeast Turkey) were released by the Company. The intercepts were high grade, close to surface and appear to be very low in contaminates. The drilling pattern was constrained to areas previously permitted for drilling. Additional diamond drilling planned in 2020, to test the extension of the mineralization, was deferred due to COVID-19 related issues.
We own 50% of the Copper Hill copper exploration prospect in a joint venture with our long-term partner, Lidya Mining. The Lidya Mining exploration team made the discovery of the Copper Hill prospect, and is now preparing to drill at the Copper Hill prospect in the 2021 summer drill season.
Turkey Regional Exploration
We hold a significant portfolio of highly prospective exploration land holdings across Turkey, some of which are progressively advancing to prospective projects. Drill testing of one of these targets, a porphyry system in western Turkey, commenced in the third quarter of 2020. Other fieldwork advanced planning for future drill testing of highly prospective gold mineralization approximately 40 km to the southwest of Çöpler.
Marigold Exploration
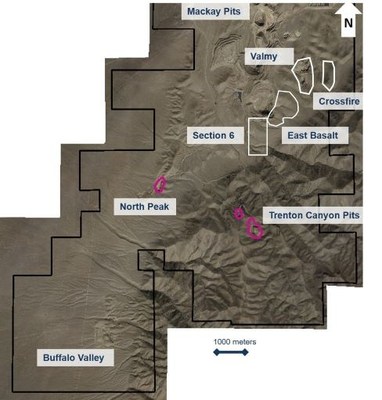
Recognizing the land limitations in advance of the previous exploration drilling program at Red Dot, we implemented a land acquisition strategy that added 11,740 hectares between 2015 and 2019.
At Valmy, there are three historic pits mined by previous owners between 2002 and 2005, which produced approximately 196,000 ounces of gold. We have been expanding Mineral Resources around these pits since acquisition in 2015. We received assays from 19 holes on Valmy targets at East Basalt and Crossfire during the third quarter of 2020, with positive results that are expected to provide Mineral Resources additions.
At Trenton Canyon, there is a historical mineral resource area and three mined pits developed by previous owners between 1996 and 2005, which produced approximately 290,000 ounces of gold. Since acquisition in 2019, we have been conducting exploration to confirm the historic drill database validity and expand known mineralization areas. The main objective is to define an open-pit oxide hosted gold Mineral Resource amenable to heap leach processing. We have received assays from 18 holes drilled on targets at West and South Pits during the third quarter of 2020, with encouraging results to support definition of Mineral Resources.
To expand the sulfide intercepts reported in May 2020, follow-up drilling indicates that this mineralization trends east-west and is inclined steeper than initial interpretation. The orthogonal thickness is believed to be 10 to 30 meters, where the reported intersected (down hole) lengths, range from 77 to 99 meters.
Predecessor companies at Buffalo Valley mined a small open pit, which produced approximately 50,000 ounces of gold between 1987 and 1990. The previous owner worked to advance the project and stated a historical estimate of 418,000 ounces of gold (20 million tonnes at an average gold grade of 0.65 g/t) in 2019. Following acquisition in mid-2019, our work has focused on verifying historical information and assessing the potential for oxide hosted gold Mineral Resources.
Geological mapping continued through the third quarter of 2020, and we completed a seismic geophysical survey south of the Basalt and Antler open pits. Once compiled, we will validate the interpretation with the current core drilling results that have identified the favourable Comus Formation beneath the property’s southern half. This work aims to establish a method of mapping the 3D structure of the main rock assemblages beneath the entire property to identify targets with potential for high-grade underground sulfide Mineral Resources.
Canada Exploration
We control two separate but contiguous claim groupings in Saskatchewan, Canada: Seabee and the Amisk project 140 km southeast of Seabee.
Seabee
Our mineral tenures comprise a 100%-owned parcel that are referred to as Seabee claims and an earn-in option parcel where we have the right to earn up to an 80% interest referred to as the Fisher Option. Since 2016, our growth and development strategy has been to increase production by optimizing the milling and mining processes and exploring new mill feed sources.
At Santoy, recent exploration success on Gap Hanging Wall (“Gap HW”) encouraged us to establish underground access to the zone on the 46 level, which is 450 meters below surface. Gap HW has excellent potential to provide additional ore feed and is approximately 220 meters in the 8A mining area’s hanging wall. Sheeted quartz veins in siliceous intrusive rock host gold mineralization at Gap HW, and the metallurgy is similar to other ores from Santoy. The excavation into the Mineral Resource is intended to help confirm structural interpretation, continuity and grades as part of our technical work to convert to Mineral Reserves. We mined 9,500 tonnes of ore, confirming continuity with grades slightly lower than block estimates.
After the temporary COVID-19 suspension, underground and surface drilling at Seabee recommenced in July and September, 2020, respectively. The focus for the third quarter of 2020 remained on infill and extension drilling of the Gap HW as well as exploring the prospective Santoy hanging wall (“Santoy HW”) target for additional resources. During the third quarter, we drilled 24 underground holes and an additional four holes from surface. We continue to receive resource grade intercepts on the Gap HW infill and step-out drilling program.
For the remainder of 2020, we intend to continue to focus on infill drilling and lateral development in support of Mineral Resource to Mineral Reserve conversion and assessment of mining methods for the Gap HW zone. Exploration to extend the area of resource grade intercepts in the Santoy HW domain is expected to continue to define further Mineral Resource additions.
The Fisher property is contiguous to Seabee claims and in May 2020, we reported encouraging drill results from gold prospects at Mac North, Yin, Abel Lake and Aurora. By the end of the third quarter of 2020, we had mobilized a drill and crew to Mac North with the objective to expand the mineralization discovered and reported earlier. Results from the planned 3,000 meter program will be released once completed.
Amisk
The Amisk property is 39,882 hectares and hosts an Indicated Mineral Resource estimate of 827,000 gold equivalent ounces (30.15 million tonnes at an average gold equivalent grade of 0.85 g/t). Proterozoic volcano-sedimentary rock assemblages, prospective for both base metal massive sulfide deposits and orogenic gold deposits, underlie the area. Our plan for this property is to investigate its potential for lode gold mineralization on the claim’s western portion. During the third quarter, detailed mapping and prospecting of the numerous gold showings on the property was completed.
Puna Exploration
There were no exploration activities at Puna during the period.
Outlook
This section provides management’s production, cost, capital, exploration and development expenditure estimates for 2020. These are “forward-looking statements” and subject to the “Cautionary Note Regarding Forward-Looking Statements”. Cash costs and AISC per ounce of gold and silver sold are non-GAAP financial measures. Please see the discussion under “Cautionary Note Regarding Non-GAAP Measures”.
On September 18, 2020, we updated our full year 2020 guidance following the successful completion of the merger with Alacer. The updated outlook also reflects the expected COVID-19 related impacts to operations at Seabee and Puna.
For fiscal 2020, we expect to produce, on a consolidated basis, 680,000 to 760,000 gold equivalent ounces from our four operating mines at consolidated AISC of $965 to $1,040 per gold equivalent ounce.
|
Operating Guidance (100%) (1)
|
|
Çöpler (2)
|
Marigold
|
Seabee
|
Puna
|
Other
|
Consolidated
|
|
Gold Production
|
koz
|
310 – 360
|
225 – 240
|
80 – 90
|
—
|
—
|
615 – 690
|
|
Silver Production
|
Moz
|
—
|
—
|
—
|
4.9 – 5.3
|
—
|
4.9 – 5.3
|
|
Gold Equivalent Production
|
koz
|
310 – 360
|
225 – 240
|
80 – 90
|
66 – 72
|
—
|
680 – 760
|
|
Cash Cost per Ounce (3)
|
$/oz
|
590 – 640
|
810 – 860
|
450 – 500
|
11.00 – 12.50
|
—
|
665 – 720
|
|
Sustaining Capital
Expenditures (4)
|
$M
|
40
|
55
|
15
|
15
|
—
|
125
|
|
Capitalized Stripping / Capitalized
Development
|
$M
|
2
|
25
|
10
|
7
|
—
|
44
|
|
Sustaining Exploration
Expenditures
|
$M
|
4
|
4
|
1
|
—
|
—
|
9
|
|
General & Administrative (5)
|
$M
|
—
|
—
|
—
|
—
|
25 – 30
|
25 – 30
|
|
Share-based Compensation (5)
|
$M
|
—
|
—
|
—
|
—
|
20 – 25
|
20 – 25
|
|
All-In Sustaining Cost per Ounce (3)
|
$/oz
|
710 – 760
|
1,170 – 1,230
|
770 – 820
|
15.00 – 17.00
|
—
|
965 – 1,040
|
|
Growth Capital Expenditures
|
$M
|
40
|
—
|
4
|
6
|
7
|
57
|
|
Growth Exploration Expenditures
|
$M
|
13
|
12
|
8
|
—
|
—
|
33
|
|
Total Growth Capital
|
$M
|
53
|
12
|
12
|
6
|
7
|
90
|
|
(1)
|
Figures may not add due to rounding.
|
|
(2)
|
Figures are reported on a 100% basis. Çöpler is 80% owned by SSR Mining.
|
|
(3)
|
We report the non-GAAP financial measures of cash costs and AISC per ounce of gold and silver sold to manage and evaluate operating performance at Çöpler, Marigold, Seabee and Puna. Refer to “Cautionary Note Regarding Non-GAAP Measures”.
|
|
(4)
|
Excludes sustaining exploration expenditures and capitalized stripping and development. Includes $9 million oxygen plant lease payment at Çöpler.
|
|
(5)
|
Figures represent the actual and projected combined expenditures and accruals for both Alacer pre-acquisition and SSR Mining for full year 2020 without considering financial reporting impacts of the acquisition. General and administrative expenses exclude share-based compensation, which is reported separately.
|
|
(6)
|
All figures in U.S. dollars, unless otherwise noted. Gold equivalent figures for 2020 operating guidance are based on a gold-to-silver ratio of 74:1. Cash costs and capital expenditures guidance is based on an oil price of $40 per barrel and an exchange rate of 1.35 Canadian dollars to one U.S. dollar and 7 Turkish lira to one U.S. dollar.
|
Production for the second half of the year is expected to be 55% to 60% weighted towards the fourth quarter due to both Seabee and Puna ramping up operations in the third quarter following COVID-19 shutdowns, stacking of higher-grade ounces later in the year at Marigold, and higher processed grades during the fourth quarter at Çöpler in line with the mine plan.
In addition to the impact of higher anticipated fourth quarter production, free cash flow generation is also expected to be heavily weighted to the fourth quarter due to the timing of the following expenditures in the third quarter:
- Transaction, integration and severance payments;
- Mine equipment and leach pad spend at Marigold;
- Tailings facility expansion spend at Seabee;
- Puna ore transportation truck purchases; and
- Puna working capital build on concentrate inventories.
Capital Returns
Capital Allocation
Our capital allocation strategy is to balance continuing investment in high-return growth, maintaining peer leading financial strength, and providing sustainable capital returns to shareholders.
In recognition of our position as a leading and sustainable free cash flow generator in the intermediate gold sector, it is our intention to return excess attributable free cash flow to shareholders through a two-tiered capital return structure. While a recurring quarterly dividend is expected to be the primary method of capital return, we will periodically evaluate supplementing this dividend from excess attributable free cash flow in the form of incremental dividends and/or share buyback programs.
Dividend Announcement and Structure
We are pleased to announce that our Board of Directors approved the initiation of a quarterly dividend (“Base Dividend”) of $0.05 per common share commencing in the first quarter of 2021. On an annualized basis, the Base Dividend represents a yield of approximately 1.0% based on the 20-day volume weighted average price as of November 11, 2020.
Periodically, the Board will consider supplementing the Base Dividend should the realized gold price remain above our Mineral Reserves gold price. Any such supplemental dividend (“Supplemental Dividend”) will be assessed using the trailing 12-month attributable excess free cash flow.
This dividend structure is intended to recognize SSR Mining as one of the highest free cash flow generating intermediate producers and provides greater alignment with the interests of shareholders in stronger gold price environments. Furthermore, we may periodically evaluate initiating a share buyback program in lieu of the Supplemental Dividend depending on prevailing market conditions and equity valuations. The declaration of any dividend is at the discretion of SSR Mining’s Board of Directors. The decision to declare a Supplemental Dividend will be based on the Company’s financial results, sustaining and growth capital investment requirements, future business outlook and other factors deemed relevant.
The dividend will be designated as an “eligible dividend” for Canadian federal and provincial income tax purposes. Dividends paid to shareholders who are non-residents of Canada will be subject to Canadian non-resident withholding taxes.
Financial and Operating Highlights
A summary of our consolidated financial and operating results for the three and nine months ended September 30, 2020 and 2019 are presented below:
|
(in thousands of US dollars, except per share data)
|
Three months ended September 30,
|
Nine months ended September 30,
|
|
|
2020
|
2019
|
2020
|
2019
|
|
Financial Results
|
|
|
|
|
|
Revenue
|
$
|
225,412
|
$
|
147,848
|
$
|
482,360
|
$
|
429,247
|
|
Income from mine operations
|
$
|
83,226
|
$
|
51,906
|
$
|
162,186
|
$
|
111,970
|
|
Gross margin (2)
|
37%
|
35%
|
34%
|
26%
|
|
Operating income
|
$
|
52,725
|
$
|
39,891
|
$
|
82,380
|
$
|
79,110
|
|
Net income
|
$
|
25,113
|
$
|
18,132
|
$
|
42,813
|
$
|
36,278
|
|
Net income attributable to equity holders of SSR
Mining
|
$
|
26,754
|
$
|
20,741
|
$
|
44,454
|
$
|
37,836
|
|
Basic attributable income per share
|
$
|
0.19
|
$
|
0.17
|
$
|
0.35
|
$
|
0.31
|
|
Adjusted attributable net income (1)
|
$
|
67,841
|
$
|
35,778
|
$
|
104,788
|
$
|
55,061
|
|
Adjusted basic attributable income per share (1)
|
$
|
0.49
|
$
|
0.29
|
$
|
0.82
|
$
|
0.45
|
|
|
|
|
|
|
|
Cash generated by operating activities
|
$
|
44,099
|
$
|
54,780
|
$
|
131,232
|
$
|
93,927
|
|
Cash generated by (used in) investing activities
|
$
|
245,106
|
$
|
(29,308)
|
$
|
234,889
|
$
|
(108,025)
|
|
Cash (used in) generated by financing activities
|
$
|
(17,077)
|
$
|
294
|
$
|
(136,266)
|
$
|
72,443
|
|
|
|
|
|
|
|
Operating Results
|
|
|
|
|
|
Gold produced (oz)
|
88,972
|
85,313
|
226,858
|
251,109
|
|
Gold sold (oz)
|
99,495
|
78,928
|
231,626
|
246,424
|
|
Silver produced (‘000 oz)
|
1,280
|
1,664
|
3,416
|
5,541
|
|
Silver sold (‘000 oz)
|
1,193
|
1,505
|
3,651
|
5,111
|
|
Lead produced (‘000 lb) (4)
|
3,952
|
5,304
|
10,664
|
15,972
|
|
Lead sold (‘000 lb) (4)
|
3,655
|
4,119
|
11,745
|
14,748
|
|
Zinc produced (‘000 lb) (4)
|
1,876
|
2,206
|
4,056
|
5,385
|
|
Zinc sold (‘000 lb) (4)
|
1,557
|
2,030
|
4,141
|
11,005
|
|
|
|
|
|
|
|
Gold equivalent produced (oz) (5)
|
106,838
|
104,775
|
267,529
|
315,622
|
|
Gold equivalent sold (oz) (5)
|
115,312
|
95,112
|
271,315
|
300,586
|
|
|
|
|
|
|
|
Average realized gold price ($/oz sold) (1)
|
$
|
1,914
|
$
|
1,480
|
$
|
1,758
|
$
|
1,364
|
|
Average realized silver price ($/oz sold) (1)
|
$
|
26.69
|
$
|
17.31
|
$
|
20.25
|
$
|
15.71
|
|
|
|
|
|
|
|
Cash cost per gold equivalent ounce sold (1, 5, 6)
|
$
|
735
|
$
|
759
|
$
|
807
|
$
|
750
|
|
AISC per gold equivalent ounce sold (1, 5, 6)
|
$
|
1,034
|
$
|
1,136
|
$
|
1,255
|
$
|
1,089
|
|
|
|
|
|
|
|
Financial Position
|
September 30, 2020
|
December 31, 2019
|
|
Cash and cash equivalents
|
$
|
733,571
|
$
|
503,647
|
|
Current assets
|
$
|
1,238,463
|
$
|
899,662
|
|
Total assets
|
$
|
5,081,054
|
$
|
1,750,107
|
|
Current liabilities
|
$
|
230,525
|
$
|
234,171
|
|
Total liabilities
|
$
|
1,276,523
|
$
|
616,153
|
|
Working capital (3)
|
$
|
1,007,938
|
$
|
665,491
|
|
(1)
|
We report non-GAAP financial measures including adjusted attributable net income, adjusted basic attributable income per share, cash costs and AISC per ounce of precious metal sold to manage and evaluate our operating performance at our mines. See “Cautionary Note Regarding Non-GAAP Measures”.
|
|
(2)
|
Gross margin is defined as income from mine operations divided by revenue.
|
|
(3)
|
Working capital is defined as current assets less current liabilities.
|
|
(4)
|
Data for lead production and sales relate only to lead in lead concentrate. Data for zinc production and sales relate only to zinc in zinc concentrate.
|
|
(5)
|
Gold equivalent ounces have been established using the average realized metal prices per ounce of precious metals sold in the period and applied to the recovered silver metal content produced by the mines. Zinc and lead production are not included in gold equivalent ounces produced.
|
|
(6)
|
Puna cash costs and AISC per silver ounce sold include a write-down of metal inventories to net realizable value of nil and $8.6 million for the three and nine months ended September 30, 2020, respectively (three and nine months ended September 30, 2019 – $1.8 million and $2.4 million, respectively).
|
Qualified Persons
The scientific and technical information contained in this news release relating to Çöpler has been reviewed and approved by Robert L. Clifford and Dr. Cengiz Y. Demirci, each of whom is a qualified person under National Instrument 43-101 – Standards of Disclosure for Mineral Projects (“NI 43-101”). Mr. Clifford is our Director, Open Pit Mine Planning and Dr. Demirci is our Vice President, Exploration. The scientific and technical information contained in this news release relating to Marigold has been reviewed and approved by Greg Gibson, P.E., and James N. Carver, each of whom is a SME Registered Member and a qualified person under NI 43-101. Mr. Gibson is our General Manager and Mr. Carver is our Exploration Manager at Marigold. The scientific and technical information contained in this news release relating to Seabee has been reviewed and approved by Samuel Mah, P.Eng., and Jeffrey Kulas, P. Geo., each of whom is a qualified person under NI 43-101. Mr. Mah is our Director, Underground Mine Planning, and Mr. Kulas is our Manager Geology, Mining Operations at Seabee. The scientific and technical information contained in this news release relating to Puna has been reviewed and approved by Robert Gill, P.Eng. and F. Carl Edmunds, P. Geo., each of whom is a qualified person under NI 43-101. Mr. Gill is our General Manager at Puna and Mr. Edmunds is our employee.
Management Discussion & Analysis and Conference Call
This news release should be read in conjunction with our unaudited Condensed Consolidated Interim Financial Statements and our MD&A as filed with the Canadian Securities Administrators and available at www.sedar.com or our website at www.ssrmining.com.
- Conference call and webcast: Thursday, November 12, 2020, at 5:00 pm EST.
- The conference call will be archived and available on our website. Audio replay will be available for two weeks by calling:
|
Toll-free in U.S. and Canada:
|
+1 (855) 669-9658, replay code 5448
|
|
All other callers:
|
+1 (412) 317-0088, replay code 5448
|
About SSR Mining
SSR Mining Inc. is a leading, free cash flow focused intermediate gold company with four producing assets located in the USA, Turkey, Canada, and Argentina, combined with a global pipeline of high-quality development and exploration assets in the USA, Turkey, Mexico, Peru, and Canada. In 2019, the four operating assets produced over 720,000 ounces of gold and 7.7 million ounces of silver.
SSR Mining’s diversified asset portfolio is comprised of high margin, long-life assets along several of the world’s most prolific precious metal districts including the Çöpler mine along the Tethyan belt in Turkey; the Marigold mine along the Battle Mountain–Eureka trend in Nevada, USA; the Seabee mine along the Trans-Hudson Corridor in Saskatchewan, Canada; and the Puna mine along the Bolivian silver belt in Jujuy, Argentina. SSR Mining has an experienced leadership team with a proven track record of value creation. Across SSR Mining, the team has expertise in project construction, mining (open pit and underground), and processing (pressure oxidation, heap leach, and flotation), with a strong commitment to health, safety and environmental management.
SSR Mining intends to leverage its strong balance sheet and proven track record of free cash flow generation as foundations to organically fund growth across the portfolio and to facilitate superior returns to shareholders.
SSR Mining is listed under the ticker symbol SSRM on the NASDAQ and the TSX, and SSR on the ASX.
SSR Mining Contacts:
F. Edward Farid, Executive Vice President, Chief Corporate Development Officer
Michael McDonald, Director, Corporate Development & Investor Relations
SSR Mining Inc.
E-Mail: [email protected]
Phone: +1 (888) 338-0046 or +1 (604) 689-3846
To receive SSR Mining’s news releases by e-mail, please register using the SSR Mining website at www.ssrmining.com.
Cautionary Note Regarding Forward-Looking Statements
This news release contains forward-looking information within the meaning of Canadian securities laws and forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 (collectively, “forward-looking statements”). All statements, other than statements of historical fact, are forward-looking statements.
Generally, forward-looking statements can be identified by the use of words or phrases such as “expects,” “anticipates,” “plans,” “projects,” “estimates,” “assumes,” “intends,” “strategy,” “objectives,” “potential,” “believes,” or variations thereof, or stating that certain actions, events or results “may,” “could,” “would,” “might” or “will” be taken, occur or be achieved, or the negative of any of these terms or similar expressions. The forward-looking statements in this news release relate to, among other things: forecasts; outlook; timing of production; our intention to return excess attributable free cash flow to shareholders; the timing and implementation of the dividend policy; the granting of any Supplemental Dividends or the implementation of any share buyback program or other supplements to the Base Dividend; statements regarding plans or expectations for the declaration of future dividends and the amount thereof; future cash costs and AISC per payable ounce of gold, silver and other metals sold; the prices of gold, silver and other metals; our ability to discover new areas of mineralization, to add Mineral Reserves, including establishing a new Mineral Reserves estimate at Gap HW at Seabee by year-end 2020, and to define additional Mineral Resources, including the discovery of additional Mineral Resources at Trenton Canyon, Valmy, East Basalt, and Crossfire; the continued construction of our new leach pad at Marigold, including the expected completion and timing thereof; the timing and extent of capital investment at our operations; the timing and extent of capitalized stripping at our operations; the timing of production and production levels and our expected drill programs at each of Marigold, Seabee and Puna, including the estimated mine life at each of these operations; production for the second half of the year being weighted towards the fourth quarter due to both Seabee and Puna ramping up operations following COVID-19 shutdowns, stacking of higher-grade ounces later in the year at Marigold and higher processed grades during the fourth quarter at Çöpler; free cash flow generation being heavily weighted to the fourth quarter due to the timing of certain expenditures; the results of the 2020 Çöpler technical report, including the timing and preliminary capital estimate for the proposed flotation circuit, the impact of the proposed flotation circuit on total sulfide plant throughput and gold production and the results of production and exploration generally; the impact of the COVID-19 outbreak on our business and financial condition, including the ability to continue operations at Seabee and Puna based on the implementation of our phased restart plans; the timing, focus and results of our exploration and development programs, including our ability to achieve certain exploration objectives at Marigold and Seabee and our ability to fast track the extension at Çöpler; Çöpler and Marigold continuing to operate with limited impact from COVID-19, including exploration activities continuing as planned; current financial resources being sufficient to carry out plans, commitments and business requirements for the next twelve months; movements in commodity prices not impacting the value of any financial instruments; estimated production rates for gold, silver and other metals produced by us; the estimated cost of sustaining capital; ongoing or future development plans and capital replacement; estimates of expected or anticipated economic returns from our mining projects, including future sales of metals, concentrate or other products produced by us and the timing thereof; and our plans and expectations for our properties and operations.
These forward-looking statements are subject to a variety of known and unknown risks, uncertainties and other factors that could cause actual events or results to differ from those expressed or implied, including, without limitation, the following: the risk that we are unable to implement the dividend policy, or that dividend payments thereunder are reduced, suspended or cancelled; uncertainty of production, development and exploration plans and cost estimates for Çöpler, Marigold, Seabee and Puna and our projects, including as a result of the COVID-19 pandemic; the continued development and resumption of operations at Seabee and Puna; disruptions to our supply chain, customers and workforce due to the COVID-19 outbreak; the responses of the relevant governments to the COVID-19 outbreak not being sufficient to contain the impact of the COVID-19 outbreak; an economic recession or downturn as a result of the COVID-19 outbreak that materially adversely affects our operations or liquidity position; the ability to successfully integrate the acquisition of Alacer; transaction, integration and severance payments in connection with the transaction, including the risk of unanticipated liabilities, unanticipated costs and the loss of key employees; our ability to replace Mineral Reserves; metal price, commodity price (including oil price) and exchange rate fluctuations; royalty expenses; political or economic instability and unexpected regulatory changes; changes in global or regional consumption, including changes in the supply or demand for precious metals and the availability and costs of metal substitutes; speculative activities; currency fluctuations; the possibility of future losses; general economic conditions; counterparty and market risks related to the sale of our concentrates and metals; uncertainty in the accuracy of Mineral Reserves and Mineral Resources estimates and in our ability to extract mineralization profitably; differences in U.S. and Canadian practices for reporting Mineral Reserves and Mineral Resources; lack of suitable infrastructure or damage to existing infrastructure; future development risks, including start-up delays and cost overruns; our ability to obtain adequate financing for mining, processing and further exploration and development programs and opportunities and for the satisfaction of payment obligations relating to our projects; uncertainty in acquiring additional commercially mineable mineral rights; delays in obtaining or failing to obtain, maintain or renew governmental permits, or non-compliance with our permits; our ability to attract and retain qualified personnel and management; the impact of governmental regulations, taxation laws and royalty rates, which may be subject to change, including health, safety and environmental regulations, including increased costs and restrictions on operations due to compliance with such regulations and the risk of non-compliance with such regulations; unpredictable risks and hazards related to the development and operation of a mine or mineral property that are beyond our control; reclamation and closure requirements for our mineral properties; potential labour unrest, including labour actions by our unionized employees at Puna, and dependence on key personnel and executives; indigenous peoples’ title claims and rights to consultation and accommodation may affect our existing operations as well as development projects and future acquisitions; certain transportation risks that could have a negative impact on our ability to operate; assessments by taxation authorities in multiple jurisdictions; recoverability of VAT and significant delays in the VAT collection process in Argentina; claims and legal proceedings, including adverse rulings in litigation against us and/or our directors or officers; complying with anti-corruption laws and internal controls, and increased regulatory compliance costs; complying with emerging climate change regulations and the impact of climate change and adverse weather conditions; the ability to fully realize the value of our shareholdings in our marketable securities, due to changes in price, liquidity or disposal cost of such marketable securities; credit risk, liquidity risk, interest rate risk, inflation rate risk and foreign currency risk; risks associated with hedging; uncertainties related to title to our mineral properties and the ability to obtain surface rights; the sufficiency of our insurance coverage; civil disobedience in the countries where our mineral properties are located; operational safety and security risks; actions required to be taken by us under human rights law; competition in the mining industry for mineral properties; our ability to select appropriate acquisition candidates, negotiate acceptable arrangements and complete and successfully integrate an announced acquisition; reputation loss resulting in decreased investor confidence; increased challenges in developing and maintaining community relations and an impediment to our overall ability to advance our projects; an event of default under our 2019 Notes may significantly reduce our liquidity and adversely affect our business; failure to meet covenants under our senior secured revolving credit facility and the Term Loan; epidemics, pandemics or other public health crises could adversely affect our business; information systems security threats; the ability to fully realize our interest in deferred consideration received in connection with divestitures; conflicts of interest that could arise from certain of our directors’ and/or officers’ involvement with other natural resource companies; uninsured risks; other risks related to our common shares; our ability to make decisions on or otherwise influence our projects that are the subject of joint ventures or are operated in conjunction with strategic partners, including the risk of delays or disputes between SSR Mining and Lidya Mining and/or Anagold; and those other various risks and uncertainties identified under the heading “Risk Factors” in our most recent Annual Information Form filed with the Canadian securities regulatory authorities and included in our most recent Annual Report on Form 40-F filed with the SEC.
This list is not exhaustive of the factors that may affect any of our forward-looking statements. Our forward-looking statements are based on what our management considers to be reasonable assumptions, beliefs, expectations and opinions based on the information currently available to it.
Assumptions have been made regarding, among other things, our ability to carry on our exploration and development activities, our ability to meet our obligations under our property agreements, the timing and results of drilling programs, the discovery of Mineral Resources and Mineral Reserves on our mineral properties, the timely receipt of required approvals and permits, including those approvals and permits required for successful project permitting, construction and operation of our projects, the price of the minerals we produce, the costs of operating and exploration expenditures, our ability to operate in a safe, efficient and effective manner, our ability to obtain financing as and when required and on reasonable terms, current financial resources being sufficient to carry out plans, commitments and business requirements for the next twelve months, our ability to continue operating at Çöpler and Marigold, our ability to resume operations at Seabee and Puna, there being no cases of COVID-19 in our workforce or any requirement for employees to self-isolate to the extent that such cases of COVID-19 impact our operations, the responses of the relevant governments to the COVID-19 outbreak being sufficient to contain the impact of the COVID-19 outbreak, long-term effects of the COVID-19 outbreak not having a material adverse impact on our operations or liquidity position, including no further temporary suspensions of operations or care and maintenance costs; the successful integration of the acquisition of Alacer; dilution and mining recovery assumptions, assumptions regarding stockpiles, the success of mining, processing, exploration and development activities, the accuracy of geological, mining and metallurgical estimates, no significant unanticipated operational or technical difficulties, maintaining good relations with the communities surrounding Çöpler, Marigold, Seabee and Puna, no significant events or changes relating to regulatory, environmental, health and safety matters, certain tax matters and no significant and continuing adverse changes in general economic conditions or conditions in the financial markets other than as set out herein (including commodity prices, foreign exchange rates and inflation rates). You are cautioned that the foregoing list is not exhaustive of all factors and assumptions which may have been used. We cannot assure you that actual events, performance or results will be consistent with these forward-looking statements, and management’s assumptions may prove to be incorrect.
Our forward-looking statements reflect current expectations regarding future events and operating performance and speak only as of the date hereof and we do not assume any obligation to update forward-looking statements if circumstances or management’s beliefs, expectations or opinions should change other than as required by applicable law. For the reasons set forth above, you should not place undue reliance on forward-looking statements.
Cautionary Note to U.S. Investors
This news release includes Mineral Reserves and Mineral Resources classification terms that comply with reporting standards in Canada and the Mineral Reserves and the Mineral Resources estimates are made in accordance with NI 43-101. NI 43-101 is a rule developed by the Canadian Securities Administrators that establishes standards for all public disclosure an issuer makes of scientific and technical information concerning mineral projects. These standards differ significantly from the requirements of the SEC set out in SEC Industry Guide 7. Consequently, Mineral Reserves and Mineral Resources information included in this news release is not comparable to similar information that would generally be disclosed by domestic U.S. reporting companies subject to the reporting and disclosure requirements of the SEC. Under SEC standards, mineralization may not be classified as a “reserve” unless the determination has been made that the mineralization could be economically produced or extracted at the time the reserve determination is made.
In addition, the SEC’s disclosure standards normally do not permit the inclusion of information concerning “Measured Mineral Resources,” “Indicated Mineral Resources” or “Inferred Mineral Resources” or other descriptions of the amount of mineralization in mineral deposits that do not constitute “reserves” by U.S. standards in documents filed with the SEC. U.S. investors should understand that “Inferred Mineral Resources” have a great amount of uncertainty as to their existence and great uncertainty as to their economic and legal feasibility. Moreover, the requirements of NI 43-101 for identification of “reserves” are also not the same as those of the SEC, and reserves reported by us in compliance with NI 43-101 may not qualify as “reserves” under SEC standards. Accordingly, information concerning mineral deposits set forth herein may not be comparable with information made public by companies that report in accordance with U.S. standards.
Cautionary Note Regarding Non-GAAP Measures
We have included certain non-GAAP performance measures throughout this document. These performance measures are employed by us to measure our operating and economic performance internally and to assist in decision-making, as well as to provide key performance information to senior management. We believe that, in addition to conventional measures prepared in accordance with GAAP, certain investors and other stakeholders also use this information to evaluate our operating and financial performance; however, these non-GAAP performance measures do not have any standardized meaning. Accordingly, these performance measures are intended to provide additional information and should not be considered in isolation or as a substitute for measures of performance prepared in accordance with GAAP. These non-GAAP measures should be read in conjunction with our condensed consolidated interim financial statements.
 View original content to download multimedia:http://www.prnewswire.com/news-releases/ssr-mining-reports-third-quarter-2020-results-and-announces-dividend-policy-301171525.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/ssr-mining-reports-third-quarter-2020-results-and-announces-dividend-policy-301171525.html
SOURCE SSR Mining Inc.